Researcher at IIT Bhubaneswar suggests neutralizing the proinflammatory function of hC5a through drug repurposing can be beneficial for COVID-19 management.
Complement and immune system are like the two sides of the coin. While activation of complement helps in cleansing the potentially infectious agents or reagents out of the human body, the process itself initiates the production of several proinflammatory mediators such as hC5a, which can trigger both acute and chronic inflammation, attributed as the “silent killer” in humans. Indeed, over stimulated complement system have been instrumental in progression of several pathophysiological conditions in humans, such as multiorgan failure, sepsis, rheumatoid arthritis, acute lung injury to cardiovascular complications, to name a few. It is noteworthy that in addition to the respiratory distress, sepsis and damage to the cardiovascular system are the prime contributor to the mortality rates in patients with COVID-19 across the globe. No wonder that >20 drug candidates targeting the various stages of the complement are currently under development by several pharma majors.
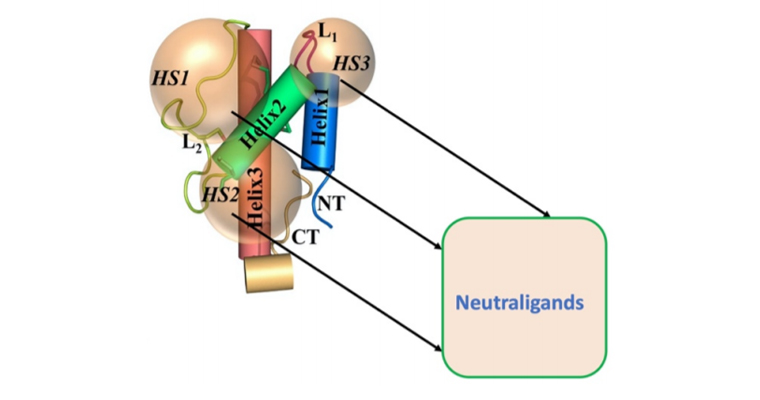
However, the idea of small molecules, directly neutralizing the function of excessive hC5a remains unexplored in the literature. In this regard, a recent study (Bioorganic Medicinal Chemistry, 2019, 27(19):115052) from the research group of Dr. S. Rana (Chemical Biology Laboratory), School of Basic Sciences, IIT Bhubaneswar have documented the first ever rational discovery of first-generation template neutraligands of hC5a through drug repurposing approach. The proof of the concept study suggests that the selected drugs perhaps bind functionally distinct hot spots (HS) on hC5a, which can be subsequently optimized as complement specific therapeutics for strongly modulating the hC5a-C5aR signalling axes and subsequently combating the COVID-19. Interestingly, Alexion’s “Eculizumab” (Soliris) is the only FDA approved anti-C5 antibody marketed since 2007, is currently undergoing careful repurposing clinical studies in USA (NCT04288713) for management of COVID-19. Similarly, InflaRx has also started its clinical trials (NCT04333420) with IFX-1, an anti-C5a antibody in Europe to combat COVID-19.

